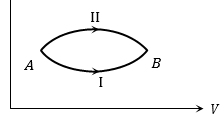
ΔU1 and ΔU2 are the changes in internal energies in the processes I and II respectively, then
(a) ΔUII > ΔUI
(b) ΔU1 < ΔU2
(c) ΔU1 = ΔU2
(d) Relation between ΔUI and ΔUII can not be determined
Ans. (c) As internal energy is a point function therefore change in internal energy does not depends upon the path followed i.e. ΔU1 = ΔU2
(160)
5/5 - (2 votes)
-
Feb 10, 2025
Click Here for Hindi medium NEET Course (252)
-
Feb 4, 2025
Best Career Counsellors in Chandigarh – Transform Your Future with Flame Institute In today’s competitive world, choosing the right career...
-
Sep 17, 2024
Best Physics coaching Teacher in Chandigarh Mohali At Flame Institute, our physics teacher is highly experienced, specializing in preparing students...
-
Sep 5, 2022
(a) – 5 J (b) – 10 J (c) – 15 J (d) – 20 J Answer-a (126)
