29. Following figure shows on adiabatic cylindrical container of volume V0 divided by an adiabatic smooth piston (area of cross-section = A) in two equal parts. An ideal gas
(Cp/Cv=γ) is at pressure P1 and temperature T1 in left part and gas at pressure P2 and temperature T2 in right part. The piston is slowly displaced and released at a position where it can stay in equilibrium. The final pressure of the two parts will be (Suppose x = displacement of the piston)
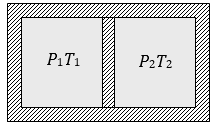
(a) P2 (b) P1
(c) [P1(V0/2)γ] / [(V0/2)+Ax]γ (d) [P2(V0/2)γ] / [(V0/2)+Ax]γ
Answer-c
(136)
5/5 - (2 votes)
-
Feb 10, 2025
Click Here for Hindi medium NEET Course (252)
-
Feb 4, 2025
Best Career Counsellors in Chandigarh – Transform Your Future with Flame Institute In today’s competitive world, choosing the right career...
-
Sep 17, 2024
Best Physics coaching Teacher in Chandigarh Mohali At Flame Institute, our physics teacher is highly experienced, specializing in preparing students...
-
Sep 5, 2022
(a) – 5 J (b) – 10 J (c) – 15 J (d) – 20 J Answer-a (126)
